Clinical Trial Supply
We are experts in global clinical trial supply,
packaging, labeling, storage, and distribution solutions
Comparator sourcing, medicines and ancillaries
Drug developers preparing for clinical trials require support to ensure that they have access to the necessary comparator and support medications, where and when patients need them.
Benefits when outsourcing clinical trials:
- Strategic savings
- Agility and flexibility
- Trial-long, uninterrupted supply
- Reduced lead times and overstock
We provide strategic insights and global, comparator sourcing solutions to support your planning and comparator sourcing teams in their efforts to set up regulatory compliant, efficient, and value-adding clinical trials.
STRATEGIC CONSULTATION
- Procurement Trends
- Emerging markets knowledge
- Cost and waste reduction
- Product Information
- Risk Analysis
SOURCING SOLUTIONS
- Global Supply Network
- Reliable Supply
- Quality Compliant
- Documentation Offered
- Specific Lots Available
PARTNERING WITH INCEPTUA FOR CLINICAL TRIALS
Our global logistics and distribution network for clinical manufacturing allows us to operate across borders and ensure supplies reach trial sites on time. We are aligned with the needs of your company and the patients who depend on us working effectively together. Inceptua’s global coverage and market leading support worldwide means one-stop control and visibility of your studies’ requirements.
Key Account Management and governance plans tailored to your needs ensure that your requirements will always be our priority.
One-Stop-Shop – Strategic Value for Clients
Sourcing – Storing – Packaging – Labeling – Distributing – Destructing
Key Strenghts
- IMP and Co-Medication Supply Chain Solutions
- Strategic Consulting from Global Expert Team
- Dedicated Resources
- Optimized Sourcing, Manufacturing and Distribution Strategy
- Quick Quotations
- No More Waiting In Line!
- Site Ordering & Inventory Management Solution
- Central Pharmacy Model
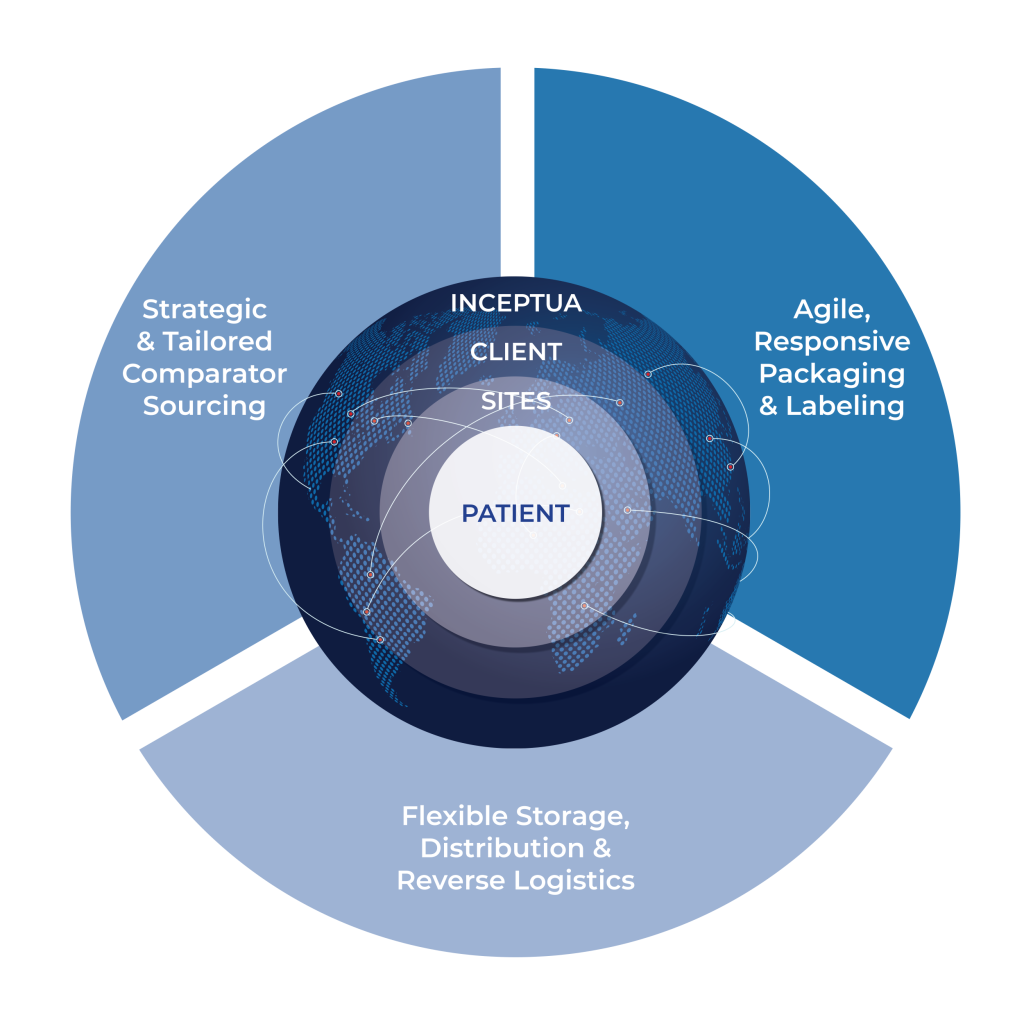
Client Value
- Strategic Cost Savings
- Reduced Lead Times and Overstock
- Agility and Flexibility
- Trial-Long, Uninterrupted Supply
- Single Strategic Vendor Managing End-to-End IMP and Co-Medication Supply Chain
Experienced, Transparent, Easy-to-Work with, providing Innovative, Agile & Responsive Solutions
Our Commitment to Sustainability
Sustainable Procurement Pledge
Mark Ware, CCO
– a Sustainable Procurement Pledge
I have endorsed the Sustainable Procurement Pledge and this is my commitment:
I spent time volunteering in Belize with the Belize Audubon Society, responsible for taking care of the country’s National Parks and the environmental health of Belize, balancing the needs of humans and nature. Taking my interest in the environment further I then gained my Masters Degree in Biodiversity, Wildlife, and Ecosystem Health from the University of Edinburgh. This passion for protecting and preserving the world around us has never left me.
Now, as Chief Commercial Officer for Inceptua I recognise the responsibility we have to continue to work responsibly, without harm to our shared environment.
Here’s my Sustainable Procurement Pledge:
I commit myself to lead by example and include sustainability as part of my overall vision and values. I will integrate sustainability into my thoughts and actions in every-day Procurement decision-making criteria and work with my colleagues and suppliers to drive lasting improvements. Likewise, when working with our partners and clients we will strive to meet their expectations for responsible Procurement and ensure that we ourselves are thoughtful providers of a sustainable service.

Mark Ware
CCO and Head of Inceptua Clinical Trial Supply
Inceptua are experts in comparator sourcing
across major and rare therapeutic areas
In 2024, Inceptua delivered over 500 medicines, benefitting over 100,000 patients across the world.
- Breast Cancer
- Colon Cancer
- Pancreatic Cancer
- Chronic Lymphocytic Leukemia (CCL)
- Hodgkin Lymphoma
- Prostate Cancer
- Wilms Tumor and other Pediatric Kidney Cancers
- Multiple Myeloma
- Testicular Cancer
- Chrohn’s Disease
- Colic
- High Cholesterol
- Blood Clots (DVT)
- Nutrition (parenteral nutrition PN)
- COVID-19
- Glucose, Saline, supplements and misc. diluents
- Fungal infections
- Thrombocytopeni
- Non-Small Cell Lung Cancer
- Myeloproliferative Neoplasms
- Acute Lymphoblastic Leukemia (ALL)
- Gastroesophageal Junction Cancer
- Kaposi Sarcoma
- Small Cell Lung Cancer
- RCC
- Meningeal Leukemia
- Cervical Cancer
- Ulcerative Colitis
- Plaque Psoriasis
- Myocardial Infarction
- Nausea
- Diabetes
- Asthma
- Tuberculosis (TB)
- Ancillaries
- Inflammation
- Other Vaccines
- Ovarian, Fallopian, or Primary Peritoneal Cancer
- Rectal Cancer
- Bladder Cancer
- Head and Neck Cancers
- Neuroblastoma / Globlastoma
- Soft Tissue Sarcoma
- HCC
- Mantle Cell Lymphoma
- Chronic Myeloid Leukemia
- Psoriatic Arthritis
- Pain indications
- Acute Transplant Rejection
- Cardiovascular Disease
- Gestational Trophoblastic Neoplasia
- Alzheimers Disease
- Schizophrenia
- Seizures
- COPD
- Acute Myeloid Leukemia (AML)
- Stomach (Gastric) Cancer
- Bone Cancer
- Bowel Cancer
- Non-Hodgkin Lymphoma
- Thyroid Cancer
- Metastatic Melanoma
- Myelofibrosis
- Arthritis
- Age-related Macular Degeneration (AMD)
- Anemia
- Macular (Dry Eye, AMD, RVO, DME, DR)
- Blood Cancer
- Brain Tumors
- Influenza
- Bacterial Infections
- Idiopathic Pulmonary Fibrosis (IFP)
20+ years’ experience in oncology comparator sourcing
Since 1997 Inceptua’s multi-national Clinical Trial Services team has been helping clients optimize their oncology clinical trial supply. We have global reach and offices across the USA, Europe, and Asia to ensure that we can offer you the right sourcing solution and essential services exactly when and where you need it, with local support.
Packaging, labelling, storage, and distribution
We support with packaging, labelling, as well as storage and distribution:
- Regional project management teams to ensure a rapid response time.
- With access to local depots in over 30 countries we can customize the supply chain to needs of your clinical trials.
- Regularly audited and qualified global partners to highest EU GxP standards
- Localized storage and distribution of Investigational and non-investigational medicinal products, as well as ancillaries.
- Frozen and controlled substance capabilities in many countries
- Return, reconciliation and destruction of excess, used and unused IMP, coordinated through a single point of contact.
MANUFACTURING SOLUTIONS
- QP Services
- Packaging Solutions
- Labeling Solutions
- GMP Certified
LOGISTICS/DISTRIBUTION
- Globally Compliant
- International Depot Network
- Customs Support
- Stock Monitoring
We have over 20 years’ experience of supplying clinical supplies to sponsors in over 50 countries, spanning all therapy areas.
We provide clinical trial supplies, from large volumes for late stage trials, to smaller quantities for pre-clinical phases. With significant experience sourcing in Europe, the Americas, and Asia, we help you find the best strategy to ensure you have access to the correct material, and an uninterrupted supply to ensure the needs of your patients are met.
Our customized solutions ensure that international borders are not a barrier to the continuation of your trial. With experience delivering into countries all over the world, our pragmatic approach to the unpredictable clinical supply chain environment will help you resolve potential issues before they even arise.
Any questions?
Ask our experts
About
Mark has 20 years of experience in clinical supplies, pre-approval access, and business development, and leads our strategic development.
EXECUTIVE VICE PRESIDENT
CLINICAL TRIAL SERVICES
CLINICAL TRIAL SERVICES
Mark Ware
About
With a background in practicing medicine, Maria oversees all activity to ensure that patient safety remains at the core of our operations.
CHIEF MEDICAL OFFICER
INCEPTUA GROUP
INCEPTUA GROUP
Maria Eklind
About
Industry expert Annika leads our global supply chain and operations, and ensures best-in-class supply chain solutions for our clients.
EXECUTIVE VICE PRESIDENT
GLOBAL SUPPLY CHAIN AND QUALITY
GLOBAL SUPPLY CHAIN AND QUALITY
Annika Kollén
About
Experienced in international business, logistics, and distribution management, Damian leads the operational activity for our Clinical Trial Services team.
OPERATIONS DIRECTOR,
CLINICAL TRIAL SERVICES
CLINICAL TRIAL SERVICES
